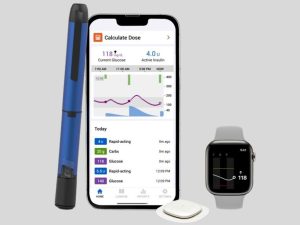
The U.S. FDA has approved the newest diabetes management innovation from Medtronic, a leader in healthcare technology: the Smart MDI system, which combines the Simplera continuous glucose monitor (CGM) with the InPen smart insulin pen. This device fills a vital gap in the treatment of diabetes by becoming the first of its type to provide missed meal dose detection and correction suggestions for multiple daily injection (MDI) users. The Simplera CGM, Medtronic’s first disposable and compact CGM device, works in tandem with the InPen app to provide real-time, personalized dosing insights. By helping users manage missed or inaccurate insulin doses, the system aims to reduce the risks associated with glucose spikes and improve overall health outcomes.
With over 40 years of pioneering innovations, Medtronic Diabetes is dedicated to alleviating the challenges of diabetes through advanced technologies, AI-powered systems, and continuous support. Headquartered in Galway, Ireland, Medtronic operates across 150 countries, employing over 95,000 professionals focused on addressing global health challenges. The company’s commitment to transforming healthcare extends to this latest launch, which begins with a limited release to current CGM and InPen customers, followed by a broader rollout. Experts hail the Smart MDI system as a breakthrough tool that simplifies insulin dosing, enhancing patient care and long-term health management.