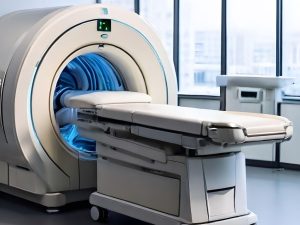
GE HealthCare has received FDA 510(k) clearance for its new dual-head SPECT/CT system, Aurora, along with its advanced Clarify DL image reconstruction technology. This system is designed to improve diagnostic precision and streamline clinical workflows. The Aurora platform integrates SPECT and CT capabilities, enabling high-quality diagnostic imaging in a single session. Its features include a 40 mm detector and 128-slice CT imaging, supporting a broad range of clinical areas such as cardiology, oncology, and neurology. The system is also compatible with various radiopharmaceuticals, including those used in theranostics. University Hospitals has become the first in the U.S. to adopt the Aurora system, reflecting its commitment to enhancing diagnostic processes.
Jean-Luc Procaccini, President and CEO, Molecular Imaging and Computed Tomography, GE HealthCare, said, “Aurora and Clarify DL are powerful reflections of GE HealthCare’s ongoing investment in next-generation imaging solutions that empower clinicians to practice precision medicine and make more informed decisions.” Aurora is also equipped with Effortless Workflow tools that aim to simplify tasks from scan preparation to completion, improving both technologist efficiency and patient comfort. This new system will be implemented alongside GE HealthCare’s broader digital solutions as part of a collaborative effort with University Hospitals to improve decision-making and care quality across the enterprise.