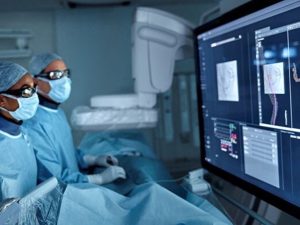
Philips, a global leader in health technology, has announced the FDA's approval of its 160cm LumiGuide endovascular navigation wire, which has been enhanced. The initial application of this new iteration of LumiGuide, which utilizes Philips Fiber Optic RealShape (FORS) technology, occurred during a complex aortic aneurysm repair procedure on a patient from the United States. The 1,000th patient to be treated with FORS technology since its introduction in 2020 is also commemorated by this milestone.
Radiation hazards are associated with conventional X-ray fluoroscopy, which is employed to guide catheters and guidewires and only provides 2D grayscale images. The Philips LumiGuide wire, which is propelled by FORS technology, facilitates a substantial improvement by allowing physicians to observe their devices in real-time in 3D and color with minimal radiation exposure. This improved visualization provides a more effective and secure method of navigating intricate procedures. Additionally, the new 160cm LumiGuide guidewire offers improved catheter compatibility and a longer reach than the current 120cm iteration, rendering it suitable for a broader spectrum of patients and procedures. It is seamlessly integrated with Philips Azurion Image Guided Therapy System, which improves procedural accuracy when used in conjunction with preoperative imaging. Philips is committed to continued innovation in image-guided therapy with this cutting-edge technology, which offers substantial advantages to both patients and physicians.